Reaction Kinetics
Reaction Kinetics
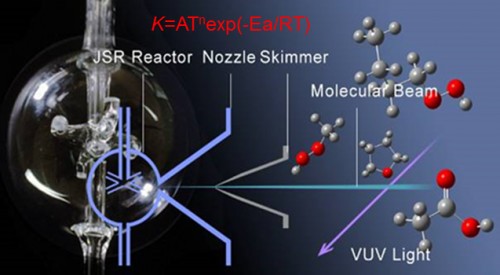
Combustion is a complex system that couple multiple physical and chemical processes, such as fluid flow, heat-transfer, mass-transfer and chemical reaction. To investigate the combustion process in deep, it is necessary to decouple these processes. Chemical reaction is a key factor that controls the combustion process, especially for the ignition, flame propagation, extinction, flammable limits, combustion stability and pollutant emission processes. The main goal of chemical kinetic study is to investigate the chemical reaction process during fuel combustion and develop reliable combustion mechanism. The developed combustion mechanism can be used for engine combustion studies and provide theoretical basis for combustor efficiency enhancement as well as pollutant reduction. This research topic focus on the combustion kinetic modeling studies of transportation surrogate fuels and their components, biofuels and other new fuels by using comprehensive experimental study, theoretical study and kinetic modeling study. Comprehensive kinetic model of the fuel will be developed, which can be reduced and used to predict the combustion processes and pollutant formations in various combustion devices.
Catalytic microkinetics
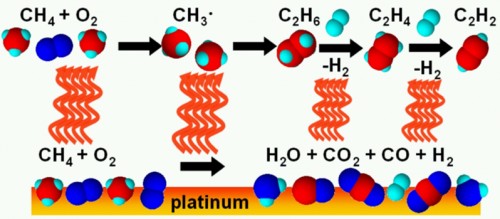
The main aim of catalytic microkinetic modeling study is to investigate the gas-solid surface catalytic reaction on molecular level and develop detailed catalytic mechanism. The mechanism can be used for industry process simulation, catalytic combustion study and fuel cell study. Moreover, the investigation of key process and intermediate in the catalytic process can provide theoretical basis for catalyst and experiment design. This research topic focus on the microkinetic modeling studies of catalytic reactions by using experimental study, theoretical study and kinetic modeling study. Reliable catalytic mechanism will be developed, which can be used for industrial catalytic process design and new catalyst design.

